How Shampoo Cleans Hair

A: Shampoo cleans hair through a fascinating process that relies on specialized molecules called surfactants. These molecules have unique properties that allow them to remove oils, dirt, and product buildup while leaving your hair clean and manageable. Let me walk you through the steps of the process.
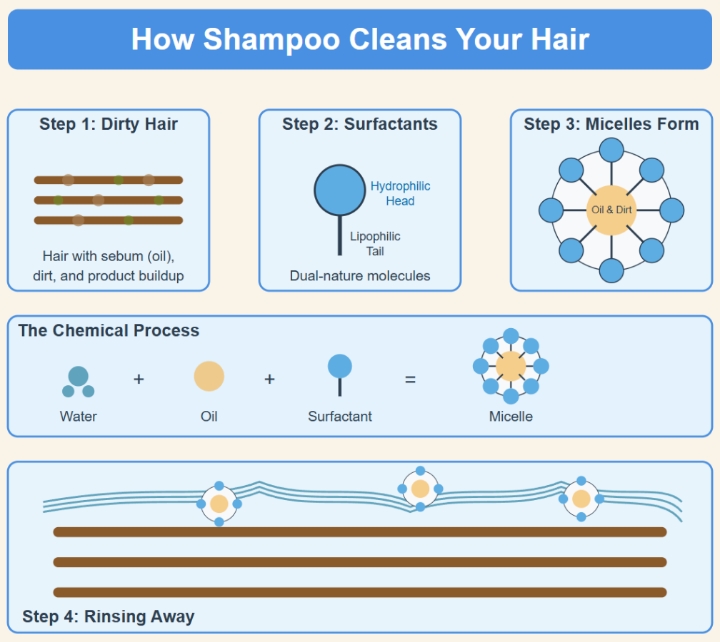
Before shampooing, your hair accumulates various substances:
• Sebum: Natural oil produced by sebaceous glands in your scalp.
• Environmental dirt: Dust, pollutants, and airborne particles.
• Product residue: Styling products, previous conditioners, and treatments.
• Dead skin cells: Natural scalp shedding.
This buildup creates a hydrophobic (water-repelling) layer on your hair. Since water alone can’t remove oils (remember how oil and water don't mix?), this is where shampoo's special chemistry comes into play.
Step 2: The Key Players - Surfactants
The cleaning power of shampoo comes from surfactants (surface active agents), which have a remarkable dual-structure:
• Hydrophilic head: The water-loving end that attracts water molecules.
• Lipophilic tail: The oil-loving end that's attracted to sebum and oils.
This dual nature allows surfactants to bridge the gap between water and oil, substances that don’t normally mix.
Common types of surfactants in shampoos include:
• Anionic surfactants: These carry a negative charge and provide powerful cleansing and foaming. They're common in most shampoos.
• Nonionic surfactants: These carry no charge and provide milder cleansing, often used in baby shampoos and products for sensitive scalps.
• Amphoteric surfactants: These can be positively or negatively charged depending on pH. They offer moderate cleansing with less irritation.
• Cationic surfactants: These carry a positive charge and are more common in conditioners than shampoos due to their conditioning rather than cleansing properties.

When you apply shampoo to wet hair, a chemical process unfolds:
1. Initial contact: Water wets the hair shaft, and you apply shampoo.
2. Micelle formation: The surfactant molecules organize themselves into spherical structures called micelles. In these structures the lipophilic tails point inward, creating an oil-friendly core and the hydrophilic heads point outward, creating a water-friendly exterior.
3. Oil capture: The lipophilic tails attract and bind to sebum, dirt, and oil from your hair and scalp, pulling them into the center of the micelle.
4. Emulsification: This critical process converts insoluble oils into a water-soluble form. The oils become surrounded by surfactant molecules, allowing them to be suspended in water rather than sticking to your hair.
5. Suspension: The micelles, now containing the trapped oils and dirt, remain suspended in the water solution due to their water-friendly outer layer.
Step 4: Rinsing - The Final Phase
When you rinse your hair, the water carries away the micelles along with their trapped dirt and oils. This process is effective because:
• The micelles are now water-soluble thanks to their hydrophilic exterior.
• The rinsing action physically removes the suspended particles from your hair.
• Fresh water continually displaces the solution containing the micelles.
The result is clean hair free from excess oils and dirt. However, this cleansing process also removes some beneficial natural oils, which is why conditioners are often used afterward to replace moisture and improve manageability.
The pH level of a shampoo plays a crucial role in determining its effectiveness and impact on hair health. The hair and scalp naturally have a slightly acidic pH, typically ranging from 4.5 to 5.5. However, many cleansing agents, or surfactants, function most effectively in slightly alkaline conditions, creating a challenge for shampoo formulation.
To address this, modern shampoos are carefully designed to balance cleansing power with pH compatibility. A well-formulated, pH-balanced shampoo ensures that the hair cuticle remains smooth and intact, preventing unnecessary swelling or damage. This, in turn, helps reduce frizz, enhance shine, and promote overall hair manageability.
Shampoo cleaning is a perfect example of chemistry in everyday life. The dual-natured surfactant molecules bridge the gap between water and oil, allowing us to effectively clean our hair. This delicate balance between cleaning power and hair health is what makes modern shampoos so effective: they remove what we don't want while preserving what we need.
The next time you lather up, you'll know exactly why those bubbles are working so effectively to clean your hair!
©Hairfinder.com
See also:
More about shampoo
How long should you keep shampoo on your hair?
Should you really change out your shampoo on a regular basis?
What happens if you apply too much shampoo
Does expensive shampoo make a difference?